You need a plan and to be able to execute it when you have to adhere to the Title 11 of the Code of Federal Regulations. Ramboll has designed digital controls systems for use in such facilities.

Control System Design
Stage 1
User Requirement Specifications
Hardware Design Specifications
System Design Specifications
Stage 2
Functional Design Specifications
Detail Design Specifications
Stage 3
Installation Qualification
Operational Qualification
Performance Qualification
With meticulous attention to detail and a commitment to excellence, we create documentation that is clear to follow and adheres to industry standards.
The correct documentation and methodology of execution is needed for the validation plan to succeed. We make sure our control system for your plant is qualified for your use.
Ramboll has executed our standard S88 methodology and control structure for 21 CFR Part 11 adherence.
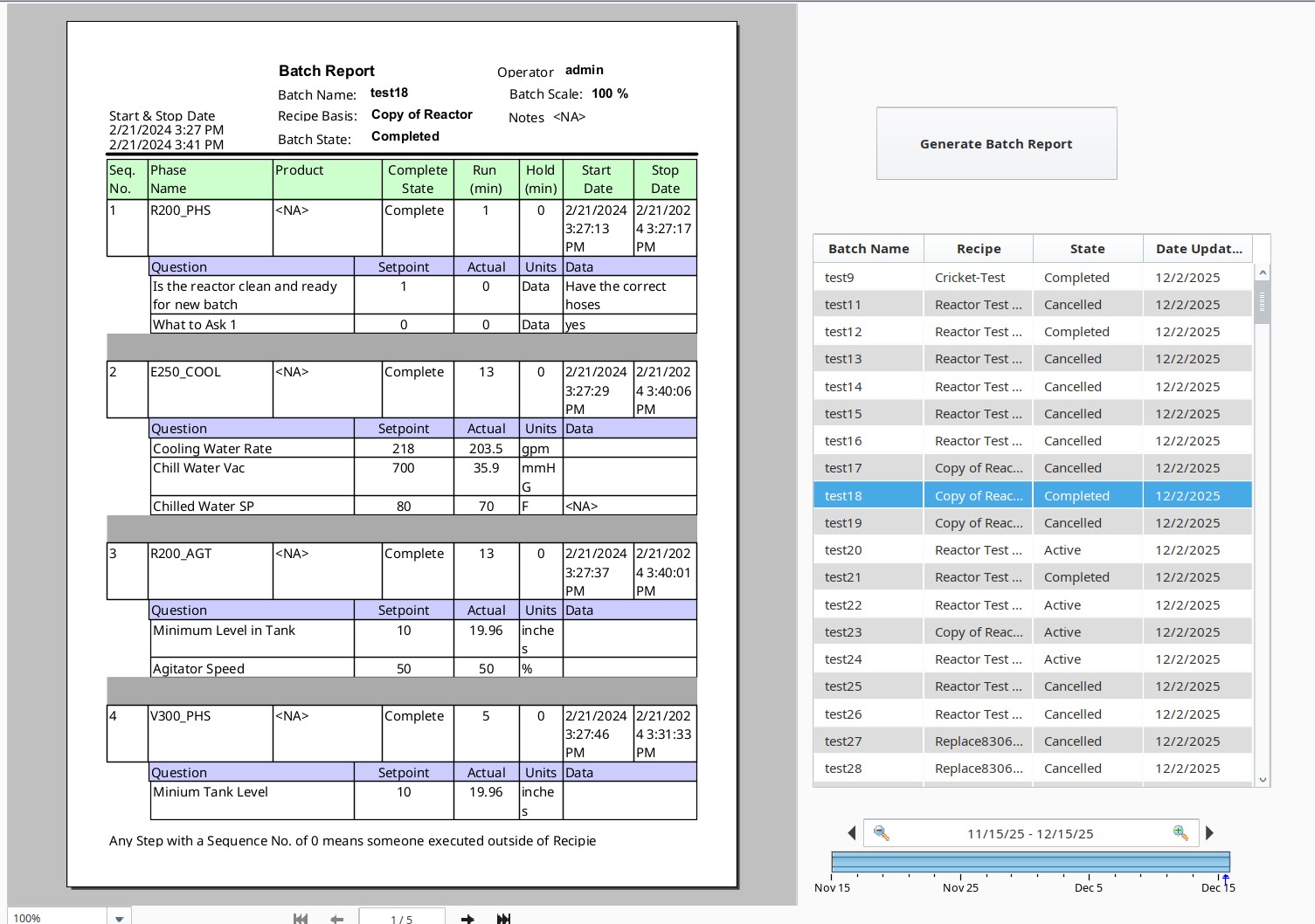
Everything is digitally Recorded
Follow what happens to each batch live or see the records as they are generated.
Our goal to make your system as efficient as possible. All records are stored in the database, which can be printed or stored as PDFs for physical hard copies.
FAQs
Is S88 valid for Pharmaceutical?
Yes, it is actually preferred since it is a methodology and documentation that can be validated and duplicated easily.
What do you record?
Our system is built to record everything. With Ignition we can even audit user interaction with the control system.
How different is it to the Standard S88 Operations?
Operationally there is minimal differences between this and our standard chemical process type operations. This system just has some extra user verification and “are you sure” moments in the paperwork.
Can you monitor and see HVAC systems?
Yes, clean rooms and HVAC systems will incorporate into the Ignition system to allow for a seamless monitoring, recording, and validation of the entire process.
Inductive Automation Ignition
- PLC/PAC agnostic
- Connect to different vendor equipment
- One Pane of Glass for whole site
